“I learned that VYJUVEK basically reintroduces a gene called COL7A1 into my wounds to help my body make the collagen VII protein.” - Emily, living with DEB
VYJUVEK delivers new COL7A1 genes directly to skin cells in DEB wounds
These COL7A1 genes restore the ability of those cells to make functional type VII collagen protein and form anchoring fibrils
Anchoring fibrils bind the inner skin layer (dermis) and outer skin layer (epidermis) together and promote wound healing
*The Open Label Extension study was designed to assess long-term safety.
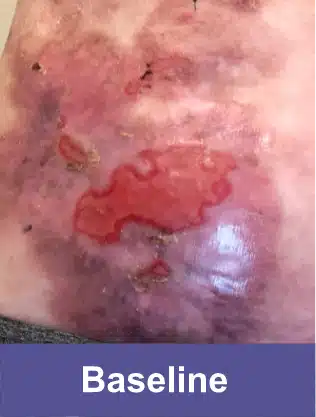
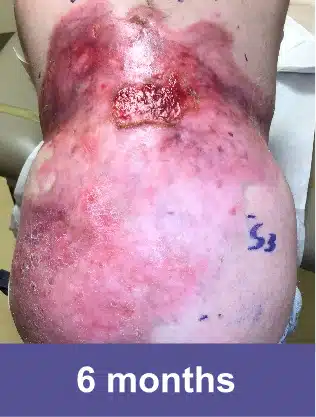
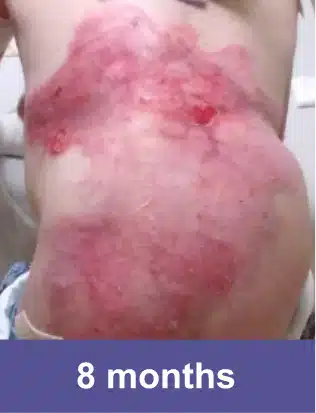
Lower Abdomen, Pediatric Female
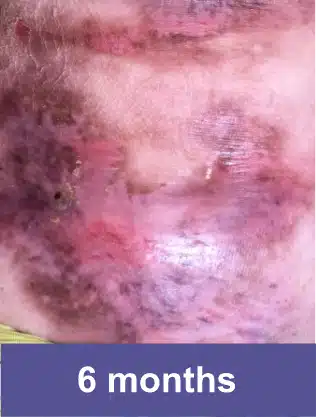
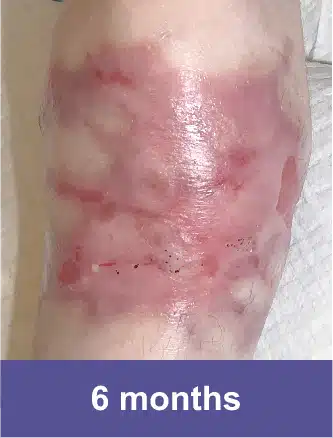
Knee, Pediatric Male
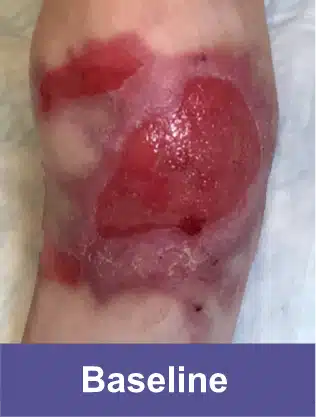
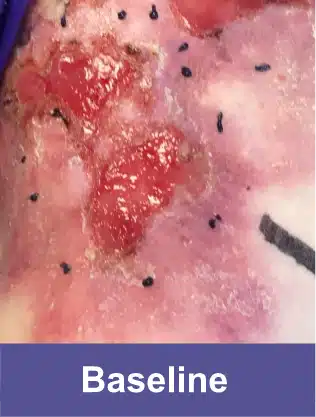
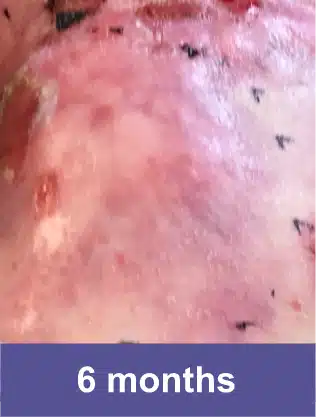
Shoulder Blade, Adult Female
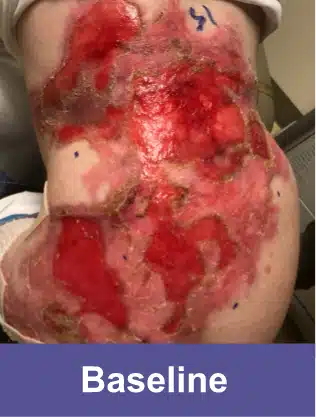
Back, Adult Male
Complete wound closure was achieved 2 months into the Open Label Extension study following completion of the GEM-3 study.‡
‡‡The Open Label Extension study was designed to assess long-term safety.
| Most common side effects in 31 people treated with VYJUVEK | |
|---|---|
| Side effects | Number of people (%) |
| Itching | 3 (10) |
| Chills | 3 (10) |
| Redness | 2 (6) |
| Rash | 2 (6) |
| Cough | 2 (6) |
| Runny Nose | 2 (6) |
VYJUVEK is a topical gel used to treat wounds in patients 6 months and older with dystrophic epidermolysis bullosa (DEB).
VYJUVEK gel must be applied by a healthcare provider.
After treatment, patients and caregivers should be careful not to touch treated wounds and dressings for 24 hours. If accidentally exposed to the VYJUVEK gel, clean the affected area.
Wash hands and wear protective gloves when changing wound dressings. Disinfect bandages from the first dressing change with a virucidal agent, and dispose of the disinfected bandages in a separate sealed plastic bag in household waste. Dispose of the subsequent used dressings in a sealed plastic bag in household waste.
Patients should avoid touching or scratching wound sites or wound dressings.
The most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose. These are not all the possible side effects with VYJUVEK. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or to the Sponsor at 1-844-557-9782.
VYJUVEK is a topical gel used to treat wounds in patients 6 months and older with dystrophic epidermolysis bullosa (DEB).
VYJUVEK gel must be applied by a healthcare provider.
After treatment, patients and caregivers should be careful not to touch treated wounds and dressings for 24 hours. If accidentally exposed to the VYJUVEK gel, clean the affected area.
Wash hands and wear protective gloves when changing wound dressings. Disinfect bandages from the first dressing change with a virucidal agent, and dispose of the disinfected bandages in a separate sealed plastic bag in household waste. Dispose of the subsequent used dressings in a sealed plastic bag in household waste.
Patients should avoid touching or scratching wound sites or wound dressings.
The most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose. These are not all the possible side effects with VYJUVEK. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or to the Sponsor at 1-844-557-9782.
You are about to leave the VYJUVEK.com website
Are you sure you want to leave?