
Learn more about VYJUVEK including wound healing results.
Download Now
An introduction to resources available to patients with DEB and their caregivers.
Download Now
A list of helpful questions to ask your doctor to have an informed discussion about VYJUVEK treatment.
Download Now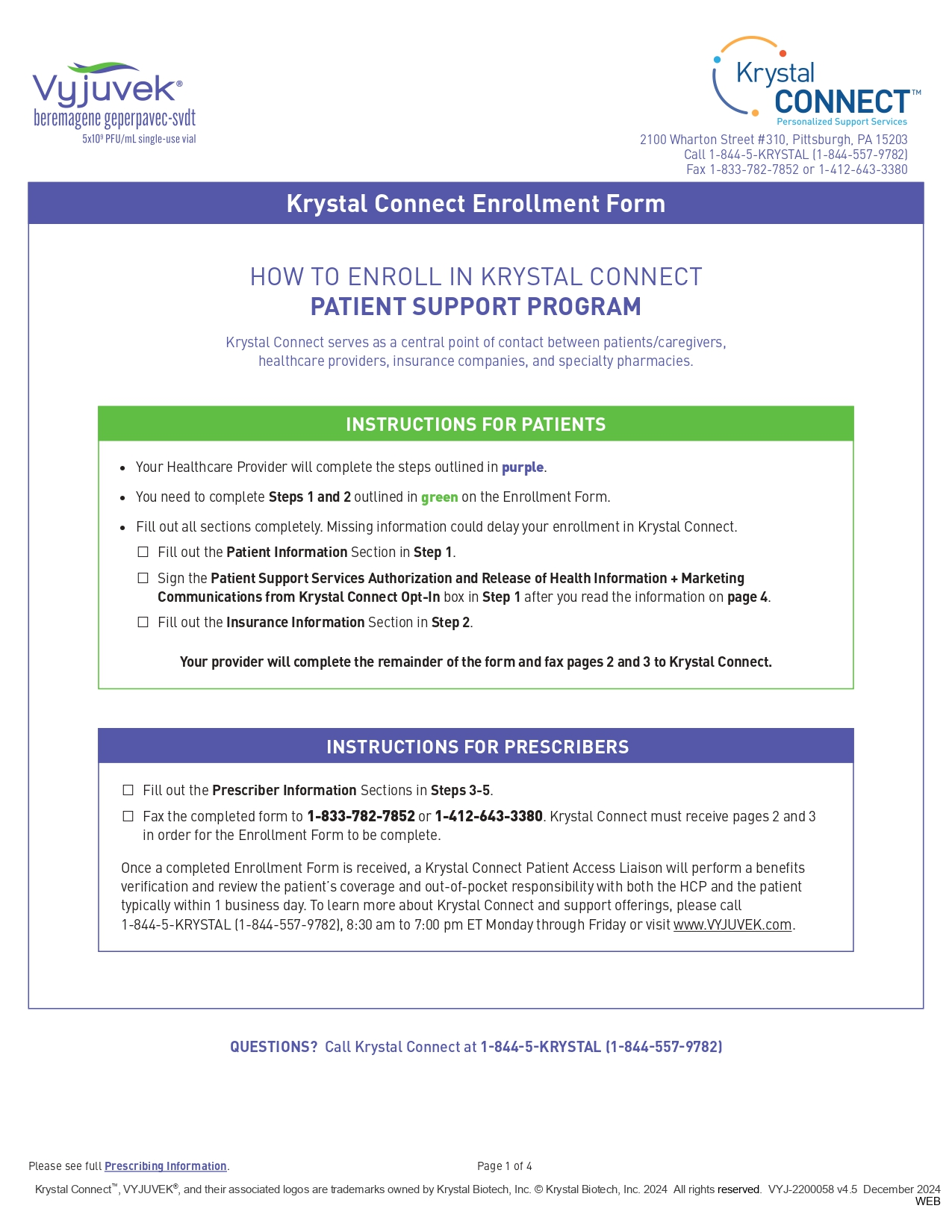
Download this form and bring it to your doctor to start your VYJUVEK prescription.
Download NowYou are not alone on your DEB journey. There are a number of organizations that provide support to individuals and families living with DEB. Here are some that might be a fit for you:
VYJUVEK is a topical gel used to treat wounds in patients 6 months and older with dystrophic epidermolysis bullosa (DEB).
VYJUVEK gel must be applied by a healthcare provider.
After treatment, patients and caregivers should be careful not to touch treated wounds and dressings for 24 hours. If accidentally exposed to the VYJUVEK gel, clean the affected area.
Wash hands and wear protective gloves when changing wound dressings. Disinfect bandages from the first dressing change with a virucidal agent, and dispose of the disinfected bandages in a separate sealed plastic bag in household waste. Dispose of the subsequent used dressings in a sealed plastic bag in household waste.
Patients should avoid touching or scratching wound sites or wound dressings.
The most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose. These are not all the possible side effects with VYJUVEK. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or to the Sponsor at 1-844-557-9782.
VYJUVEK is a topical gel used to treat wounds in patients 6 months and older with dystrophic epidermolysis bullosa (DEB).
VYJUVEK gel must be applied by a healthcare provider.
After treatment, patients and caregivers should be careful not to touch treated wounds and dressings for 24 hours. If accidentally exposed to the VYJUVEK gel, clean the affected area.
Wash hands and wear protective gloves when changing wound dressings. Disinfect bandages from the first dressing change with a virucidal agent, and dispose of the disinfected bandages in a separate sealed plastic bag in household waste. Dispose of the subsequent used dressings in a sealed plastic bag in household waste.
Patients should avoid touching or scratching wound sites or wound dressings.
The most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose. These are not all the possible side effects with VYJUVEK. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or to the Sponsor at 1-844-557-9782.
You are about to leave the VYJUVEK.com website
Are you sure you want to leave?