INDICATION AND USAGE
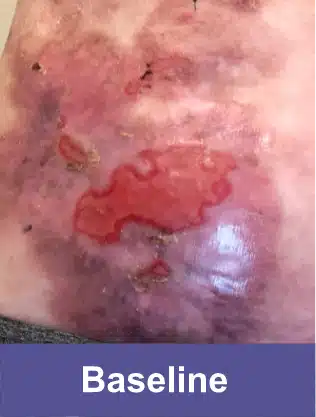
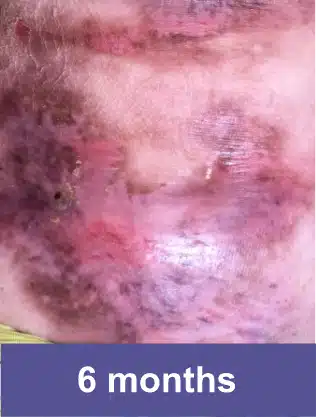
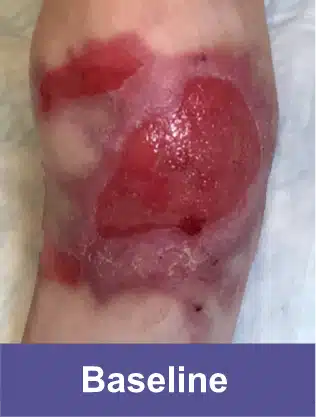
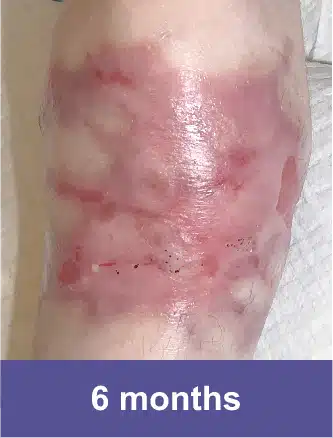
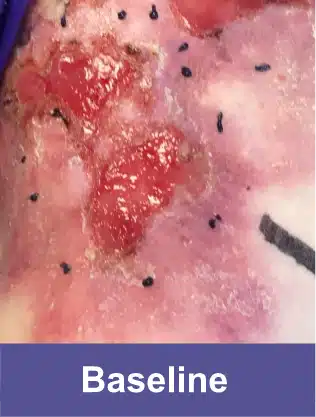
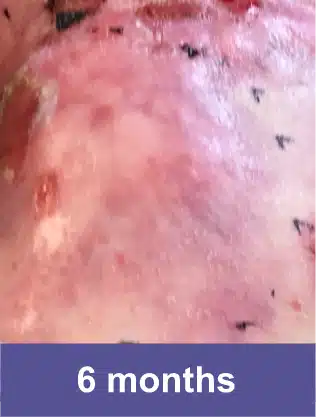
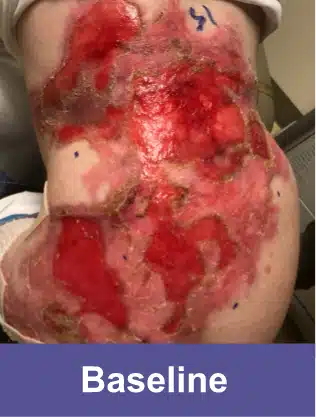
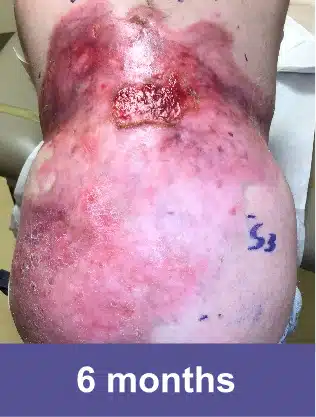
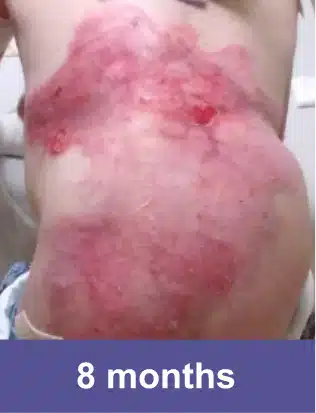
VYJUVEK is a topical gel used to treat wounds in adult and pediatric patients (from birth) with dystrophic epidermolysis bullosa (DEB).
IMPORTANT SAFETY INFORMATION
VYJUVEK gel should be applied by a healthcare professional, patient, or caregiver.
After treatment, patients and caregivers should be careful not to touch treated wounds and dressings until the next bandage change. If accidentally exposed to the VYJUVEK gel, clean the affected area.
Wash hands and wear protective gloves when changing wound dressings. Disinfect bandages from the first dressing change with a virucidal agent and dispose of the disinfected bandages in a separate sealed plastic bag in household waste. Dispose of the subsequent used dressings in a sealed plastic bag in household waste.
The most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose. These are not all the possible side effects with VYJUVEK. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or to the Sponsor at 1-844-557-9782.
- Please see Important Safety Information above and click here for full Prescribing Information.